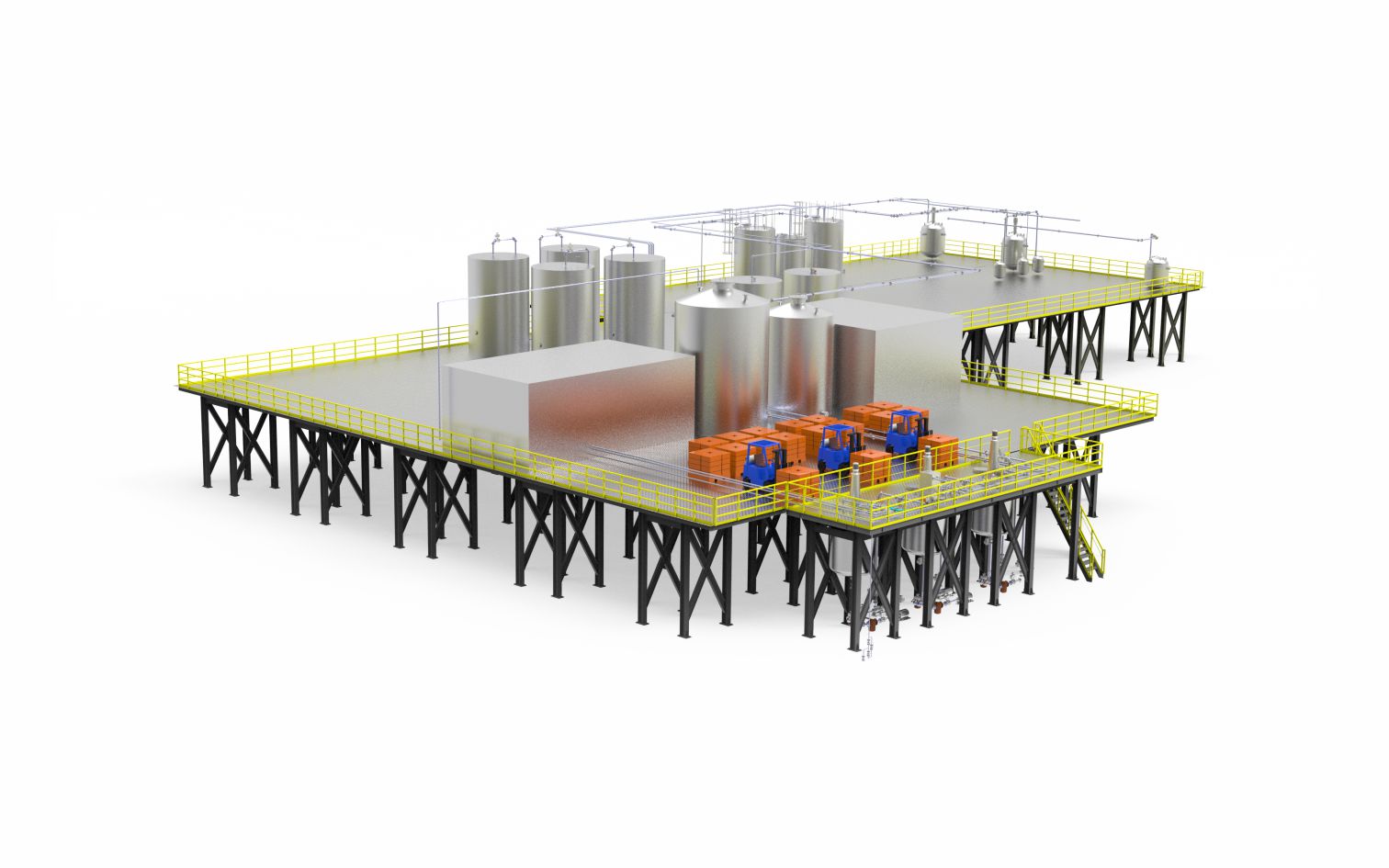
SOPHON provides comprehensive and one-stop solutions fully covering the entire process for industries including food, healthcare, cosmetics, and pharmaceuticals. The complete production line strictly adheres to three core standards: precision ingredient control, guaranteed quality consistency, and intelligent production management. It integrates key modules such as the feeding system, grinding system, drying system, pre-firing system, and automated control system. Through deep collaboration between the intelligent production management system and digital control programs, the entire workflow—from raw material intake to finished product inspection and packaging storage—is scientifically interconnected, ensuring orderly coordination and highly efficient operation across all production stages.
From dust-free unloading and intelligent screening & impurity removal in the raw material pretreatment stage to automated packaging and end-to-end quality inspection in the finished product stage, SOPHON's automated production line not only complies with stringent cleanroom production standards, but also, through equipment intelligence upgrades and process parameter optimization, helps enterprises achieve a production efficiency increase of over 30%. It serves as a bridge for the transformation from traditional manufacturing to digital and intelligent production, providing an integrated solution that combines technological foresight and practical applicability for high-end manufacturing sectors.